First assignment on acids and bases
1. The color of the solution determines if it is an acid, base, or neutral solution.
A. TrueB. False
C. Pink are Base and clear are Acid.
Answer: is B, False. The color of the solution cannot determine if a solution is acid or base. The pH is the determining factor of acids and bases. In order to tell if one is acid or base you need to get a pH reading. If the reading is below 7 the solution is an acidic solution, if the pH is above 7 it is a basic solution. If the solutions pH is exactly 7 it is considered a neutral solution.
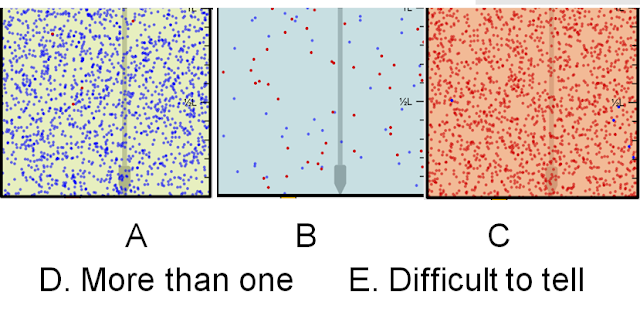
2. Which solution is Basic?
Answer: D. More than one. This answer is correct because both B and C's pH's are above 7.
3. Which Solution is Acidic?
Answer: C. The reason that you can tell is the extremely high level of H30+.
4. Which solution is Basic?
Answer: B. You can tell this easily by looking at the OH- levels. A's levels are equal so it is a neutral solution, B's levels show that the OH- levels are very high, meaning it is basic, C's levels show that the H30+ levels are very high, making it acidic. Additional ifno:
5. Which Solution is acidic?
Answer: D. This can be explained by looking at H3O+, the neutral solution for H3O+ is 10^16, so both A and B are acidic.
6. How will adding water effect the pH?
Answer: A, adding water to the solution will dulute it, causing the high acidic solution to become less concentrated, because waters pH is 7 the pH of the solution that is 5 will go up.
7. How will equal amounts of water effect the pH?
Answer: B. Adding water to the already Basic solution will decrease the pH, because the solution is very high in basitity adding water (which has a pH of 7) will dillute the solution and lower the pH.
8. What is the order from most Acidic to most Basic?
Answer: A, because the pH of all 3 increase in that order.
9. What is the order from most Acidic to most Basic?
Answer: C. This is explained because right off the bat you know that A is Neutral so it will be in the middle, then you look at B, from looking at B you can see the high amount of OH- from looking at this you can tell that it is a very acidic solution so if we are going from Acidic to Basic then B would be the first one in the line up.
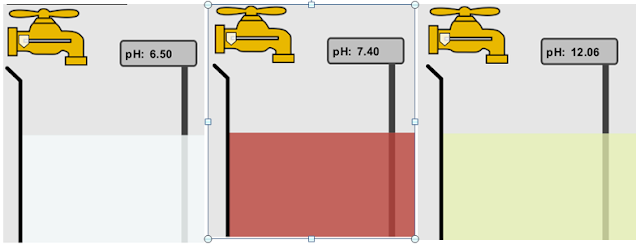
10. If spit has a pH of 7.4,what does that tell you about the water equilibrium?
6. How will adding water effect the pH?
Answer: A, adding water to the solution will dulute it, causing the high acidic solution to become less concentrated, because waters pH is 7 the pH of the solution that is 5 will go up.
7. How will equal amounts of water effect the pH?
Answer: B. Adding water to the already Basic solution will decrease the pH, because the solution is very high in basitity adding water (which has a pH of 7) will dillute the solution and lower the pH.
8. What is the order from most Acidic to most Basic?
Answer: A, because the pH of all 3 increase in that order.
9. What is the order from most Acidic to most Basic?
Answer: C. This is explained because right off the bat you know that A is Neutral so it will be in the middle, then you look at B, from looking at B you can see the high amount of OH- from looking at this you can tell that it is a very acidic solution so if we are going from Acidic to Basic then B would be the first one in the line up.
10. If spit has a pH of 7.4,what does that tell you about the water equilibrium?
Answer: A. This can be explained because spit is essentially water with something added. When that extra something was added it caused the balance of the solution to change, which also changes the pH. This being added has caused the equilibrium to shift left.













No comments:
Post a Comment